To make clinical trials faster, less costly and more successful, Biopharmaceutical companies are moving from fixed to flexible study designs.
Oncology - The area with lowest likelihood of approval (LOA)
A recent report, the largest ever study of clinical development success rates, analyzed 9,985 clinical and regulatory phase transitions, across 1,103 companies and concluded that of the 14 major disease areas Oncology had the lowest likelihood of approval (LOA) with just 5.1%.
Now is the time to move from fixed to flexible trial designs
New adaptive methodologies are far more flexible than traditional designs as they allow trialists to monitor the flow of information and adjust the trial without undermining the integrity and validity of the trial.
It is said that the negative impact of high uncertainty at design stage for a confirmatory trial can be mitigated by an sample size re-estimation design.
Sample Size Re-Estimation for Oncology Trials
A sample size re-estimation (SSR) design is a flexible, adaptive design with the primary purpose of allowing sample size of a study to be reassessed in the mid-course of the study to ensure adequate power. Statistical analysis plans for adaptive trials should cover interim analyses to optimise efficiency with the final analyses and to draw final conclusions about the observed treatment differences.
An interim sample size reassessment aims to ensure sufficient power, adaptation of the allocation ratio to ensure more patients receive the superior treatment, dropping of inferior treatments, addition of new treatment arms to save time and resources, population “enrichment” to narrow scope of the clinical trial, or transition directly from one trial phase to another.
Source BMJ: Key design considerations for adaptive clinical trials: a primer for clinicians

What are the advantages of Sample Size Re-estimation?
- Earlier Decisions
You inherit the Group Sequential Design which allows the researcher to stop early for efficacy or futility. This allows the ability to adjust the trial to the reality of what the effect size is telling you will happen in the trial.
- Reduced Potential Cost
By having the ability to stop a trial early, you inherit the reduced costs that come with this, such as not investing money in a futile trial.
- Higher Potential Success
In an unblinded sample size re-estimation you have a wider range of effect sizes. In contrast to the traditional case you are now given the ability to increase the sample size for those relevant effect sizes that are lower than you initially planned for.
- Greater Generalizability
There’s a wider range of potential effects and this optionality might give you the ability in more complex unblinded sample size re-estimation to test additional treatments.
- Stakeholder Buy-in
Optionality can make up-front investment lower, which will appeal to stakeholders. There is also a better chance of success valuable to subjects.
How to increase the success of Oncology clinical trials
A recent paper featured in Applied Clinical Trials recommends the following three steps for advancing oncology drug development:
- Selecting the best drug candidates
- Identifying and eliminating failures as early as possible
- Designing trials to identify the right dose, for the right disease, in the right patients as early as possible.
It is accepted that a very low number of drugs in development will be successful in demonstrating efficacy - so earlier information and better-focused evaluation are critical to improving success rates. This is where sample size re-estimation can help.
Unblinded Sample Size Re-estimation
with Interim Monitoring for Two Survival Curves
- New nQuery Adaptive Function - Arriving May 2019 -
Led by customer demand and the need to overcome the difficulties oncology trials face, the May 2019 release of nQuery Advanced (version 8.4) will include a new section dedicated to unblinded sample size re-estimation with interim monitoring for survival analysis.
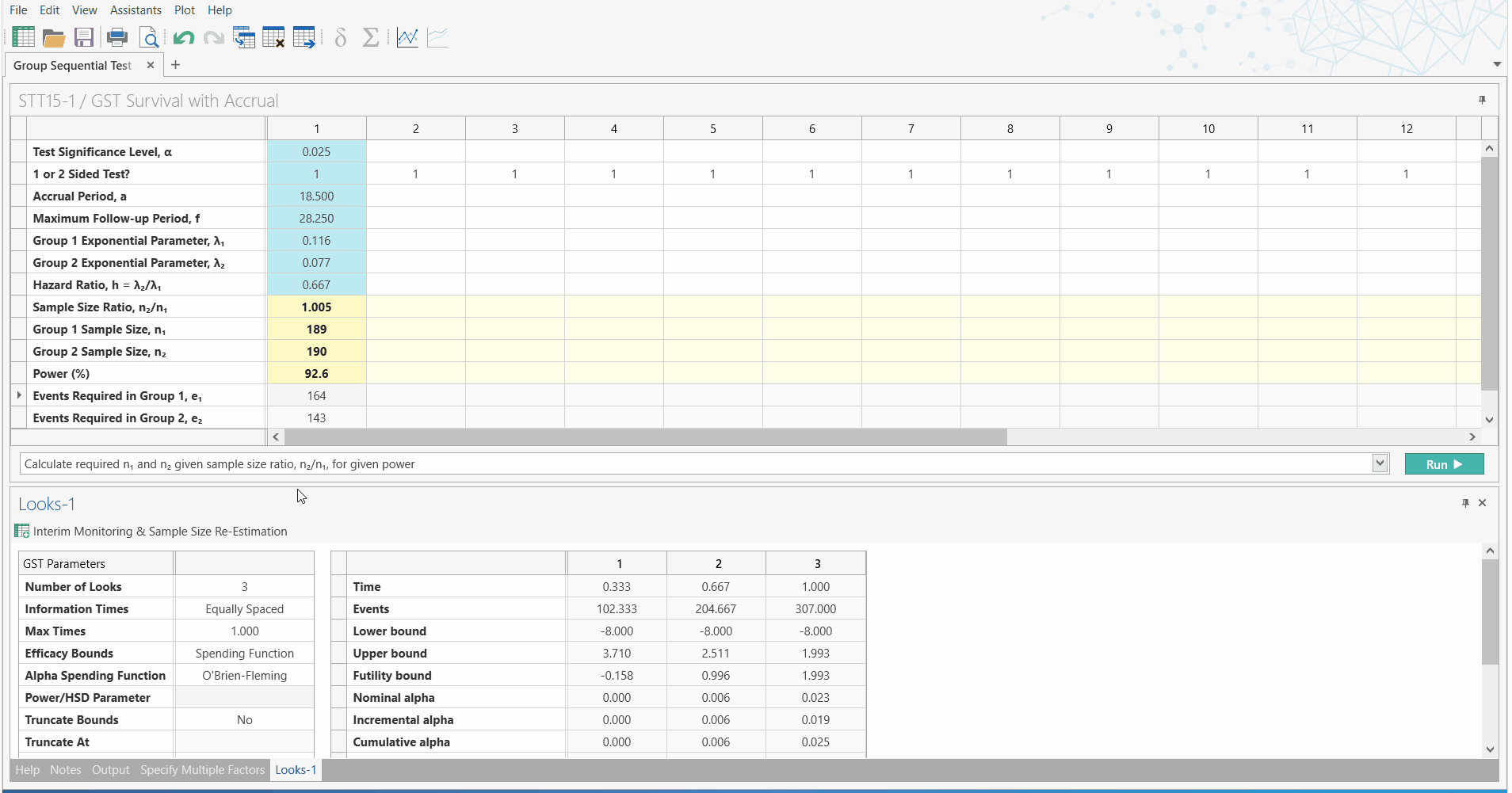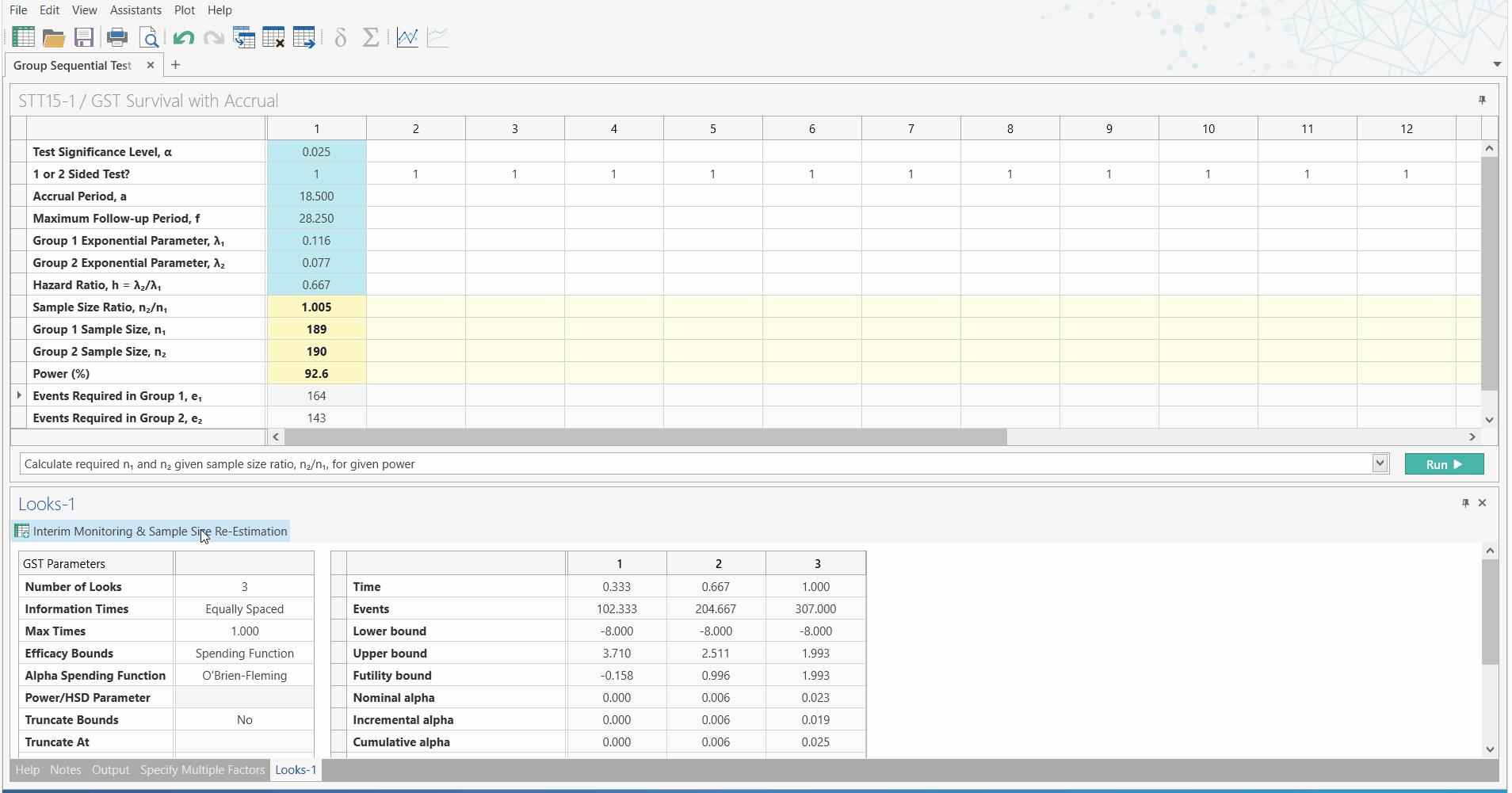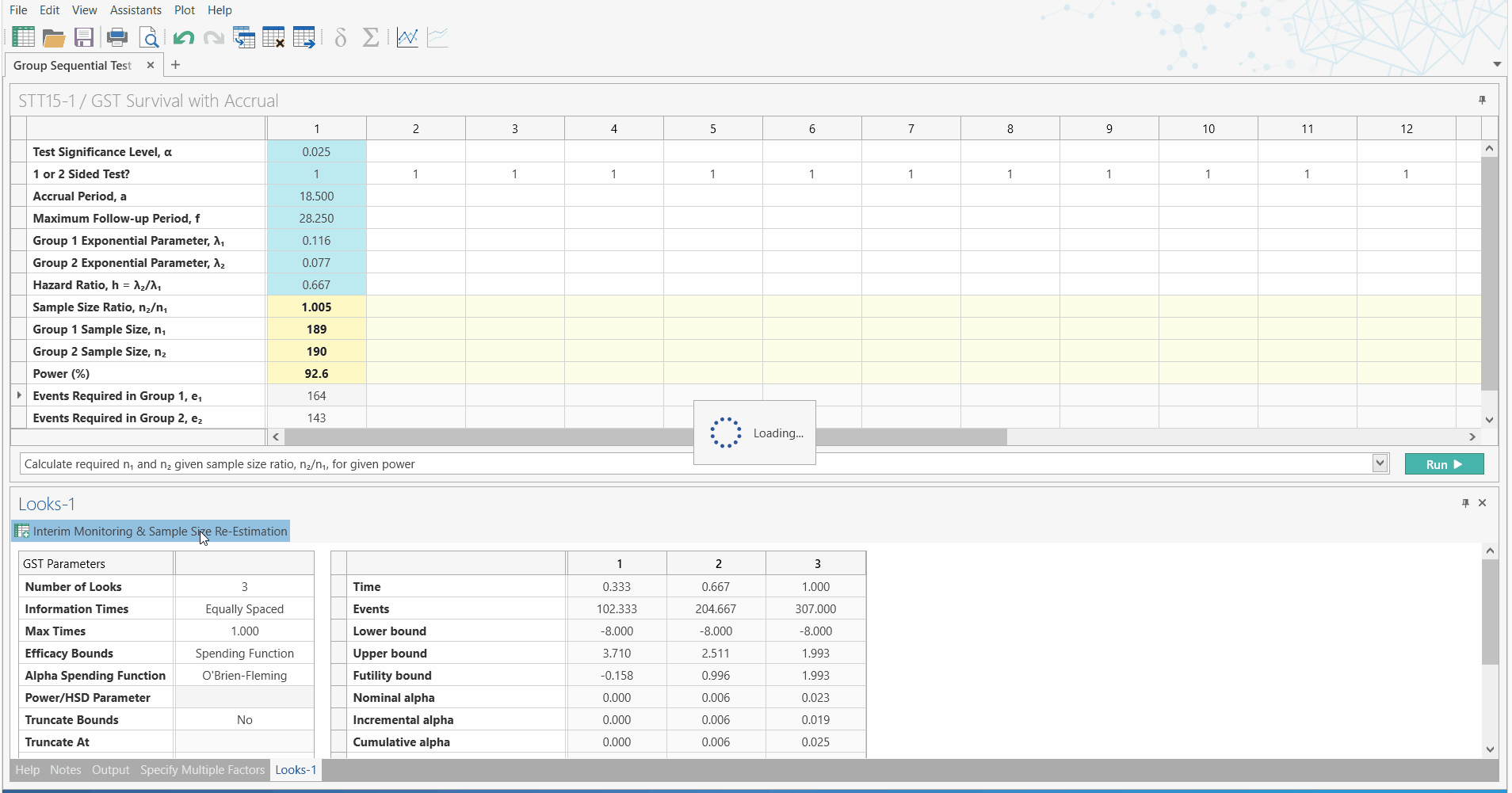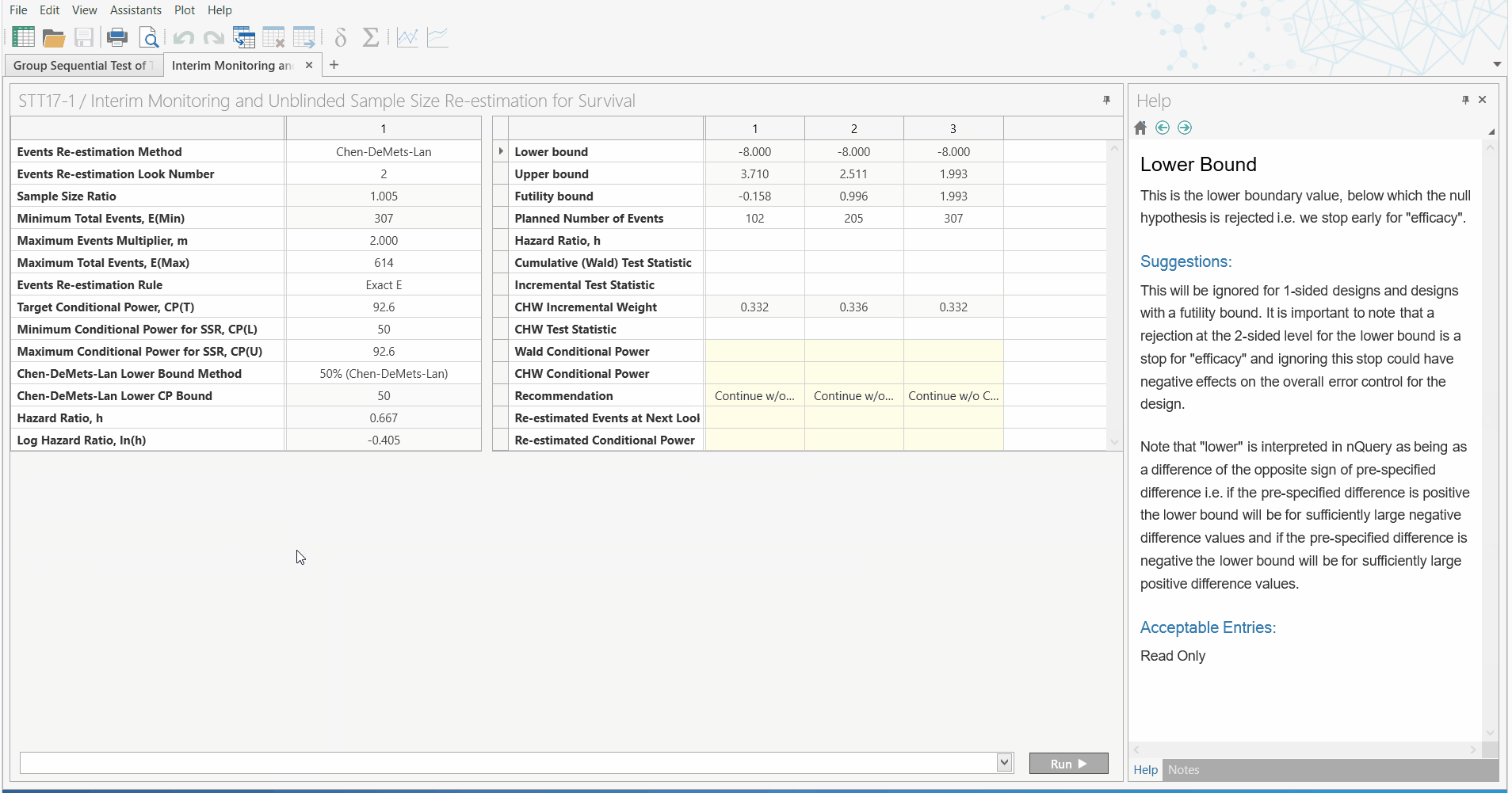
So what does this mean for Biostatisticans? With this addition to nQuery, Biostatisticians can easily conduct sample size re-estimation for survival analysis, using a nQuery - the highly trusted and validated sample size platform. Using the familiar and easy to use spreadsheet interface, researchers can now quickly improve their research capabilities.
Find out more about Adaptive trials here.




















No Comments Yet
Let us know what you think