What is Simon's Two-Stage Design?
Simon's Two-Stage Design is a type of phase II clinical trial. It is one of the most common multi-stage designs used in Phase IIa clinical trials. The Simon two-stage design is an exact design which allows flexibility regarding the null and alternative hypotheses while also allowing stopping for futility.
The objective is to try and establish whether the proportion of responses is sufficiently high to recommend this drug to go to the next step in the clinical trial phase, Phase IIB.
Below is a video extract from our webinar Sample Size for Phase II Clinical Trials.
After you have watched the video, read on for more information on Simon's Two Stage design.
How does Simon's Two-Stage design work?
Phase IIa designs are focused on proof-of-concept showing the potential efficacy and safety of a proposed treatment. Multi-stage designs are common to allow for flexibility to stop trials early for futility as Phase II is the most common failure point in drug evaluation.
With Simon's design, a trial is conducted in two stages, with the option to stop the trial after the first or after the second stage. The basic approach is to “minimize” expected sample size when the true response is low.
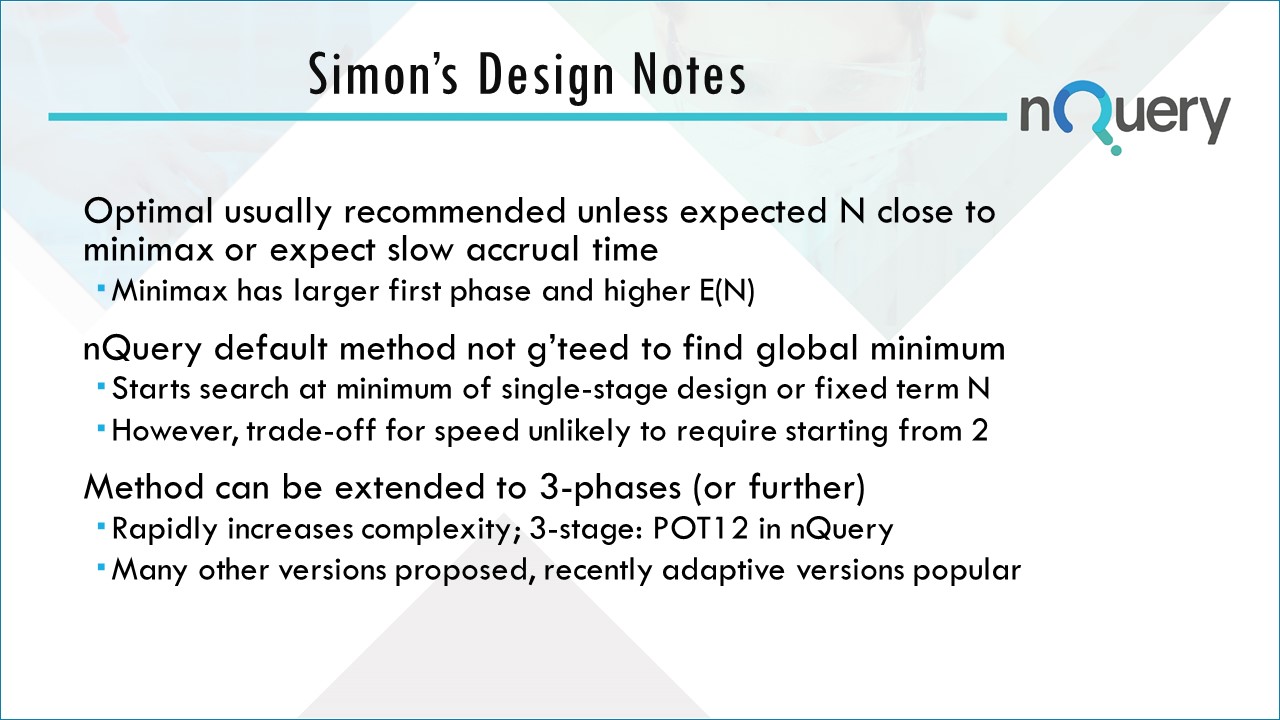
The Simon two-stage approach involves two criteria, minimax and optimal, for selecting sample sizes and critical values for these two-stage designs.
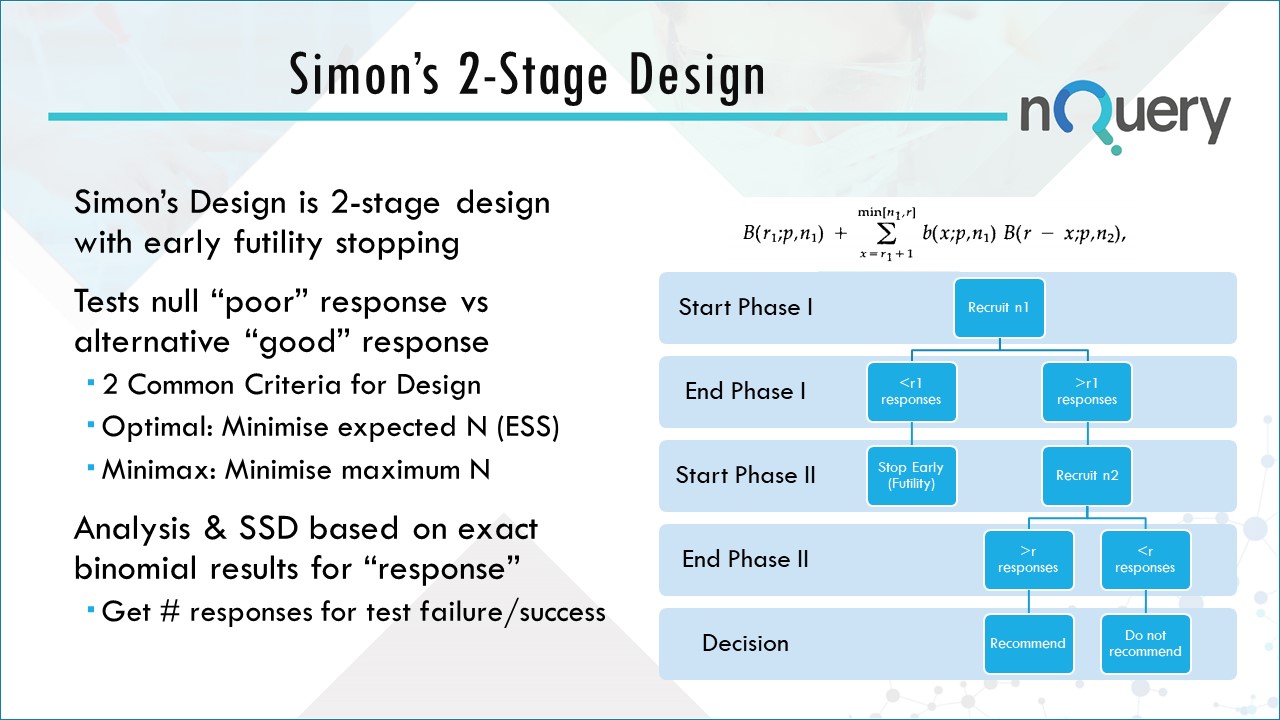
Usually, the minimax two-stage design has the same maximum sample size n as the smallest single-stage design that satisfies the error probabilities. However, because of the early termination option (after first stage), the minimax two-stage design has a smaller expected sample size under H0.
Why is Simon's two-stage design so widely used?
Simon’s two-stage designs have obvious advantages over the conventional single stage design.
- Considerable savings in expected sample sizes can be achieved if the alternative hypothesis is in fact true, without sample sizes suffering too much if the null hypothesis is true.
- From ethical and economical considerations, a trial should be allowed to stop earlier after an interim analysis to better protect patients, especially in situations when the treatment is not effective.
- Will minimize the number of patients treated with an ineffective drug.
- It extends knowledge of toxicology and pharmacology of drug/agent.
How do I learn more about Simon's two-stage design?

If you are interested in learning more about Simon's Two-Stage Design, check out our webinar, Sample Size for Phase II Clinical Trials Simon's Design and MCP-Mod Case Studies






















No Comments Yet
Let us know what you think