What are the advantages & disadvantages of unblinded sample size re-estimation in clinical trials? Let us quickly examine them and what they mean for you.
Adaptive trials are any trial where a change or decision is made to a trial while the trial is still on-going. It is said that trials with an adaptive design are often more efficient, informative and ethical than trials with a traditional fixed design since they often make better use of resources such as time and money and may require fewer participants.
There are many types of adaptive trials designs. One popular type is that of sample size re-estimation (SSR). A sample size re-estimation (SSR) design is a flexible, adaptive design with the primary purpose of allowing sample size of a study to be reassessed in the mid-course of the study to ensure adequate power.
In the real world, many presumptions at the planning phase are made for the sample size calculation, namely the effect size and any additional “nuisance” parameters (e.g., variability associated with primary outcome; control group event rate; accrual rate). These can have a level of uncertainty. This uncertainty at design stage for a confirmatory trial can be mitigated by an sample size re-estimation (SSR) design.

What is unblinded sample size re-estimation in clinical trials?
There are two primary types of (SSR) sample size determination. These are:
- Unblinded Sample Size Re-Estimation
- Blinded Sample Size Re-Estimation
This blog will focus on Unblinded Sample Size Re-Estimation. View our blog post on the advantages and disadvantages of blinded sample size re-estimation to learn about blinded SSR.
The Advantages & Disadvantages of
Unblinded Sample Size Re-Estimation in Clinical Trials
This video is an excerpt from our webinar The Advantages & Disadvantages of Adaptive Sample Size Re-Estimation.
The advantages of unblinded sample size re-estimation clinical trials
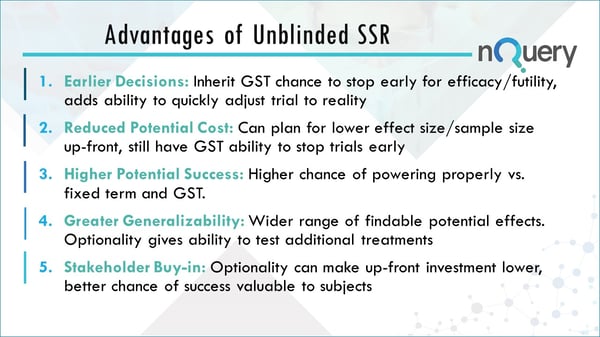
Earlier Decisions
You inherit the Group Sequential Design which allows the researcher to stop early for efficacy or futility. This allows the ability to adjust the trial to the reality of what the effect size is telling you will happen in the trial.
Reduced Potential Cost
Researchers can plan for a lower effect size or sample size to begin with but will still have the ability to stop the trial early and change this. You do not have to increase the sample size, however, you now have the option to. You may save a trial that is not on the right trajectory by using this approach. Also by having the ability to stop a trial early, you inherit the reduced costs that come with this, such as not investing money in a futile trial.
Higher Potential Success
In an unblinded sample size re-estimation you have a wider range of effect sizes which are now within the plausible success range. This is in contrast to the traditional case because you now are given the ability to increase the sample size for those relevant effect sizes that are lower than you initially planned for.
Greater Generalizability
There’s a wider range of potential effects and this optionality might give you the ability in more complex unblinded sample size re-estimation to test additional treatments.
Stakeholder Buy-in
Optionality can make up-front investment lower, which will appeal to stakeholders. There is also a better chance of success valuable to subjects.
The risks of unblinded sample size re-estimation clinical trials
Like any trial design, if not executed properly there can be some disadvantages. Below is a quick summary of the potential disadvantages of unblinded sample size re-estimation (SSR). However there are ways to overcome these with careful planning.
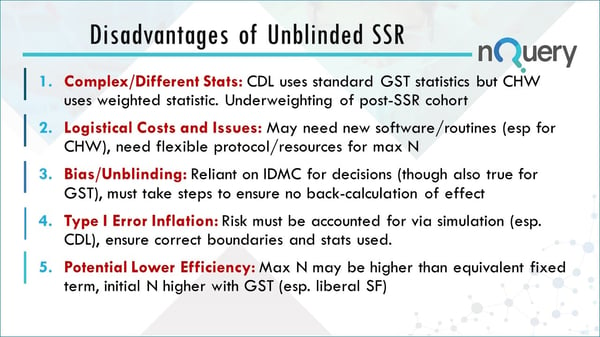
Complex/ Different Stats
The Cui, Hung & Wang approach uses weighted statistics which makes it not directly comparable. This approach is underweighting the post sample size re-estimation cohort because it will be based on the initial sample size proposed not the actual one.
Logistical Cost and Issues
There may be a need for new software and routines, particularly for the CHW. There will also need to be additional planning for having a more flexible protocol and maybe additional resources if your sample size is doubling.
Bias/Unblinding
Bias and unblinding is a major issue. The researcher will rely on IDMC for decisions. You must take steps to ensure that no one can back calculate the effect size. This is particularly true if you’re only going to increase the sample size up to a very specific point to restore the conditional power to the target additional power, then someone could back calculate the effect size. This is why you shouldn’t let too many people know what the total sample size has been increased to if it isn’t necessary.
Type I error inflation
The risk must be accounted for via simulation. You should ensure that you know the correct statistics being used for your particular design and that you’re not breaking the rules of what’s allowed.
Potential Lower Efficiency
The maximum n that you may have for this type of trial may be higher than you would have had for an equivalent fixed term or group sequential trial. You may have spent more resources than you would have in an equivalent group sequential trial. There is a lot of debate regarding the efficiency gains of both these approaches.
Like any trial design careful planning can mitigate or eliminate certain risks.
References:
Sample Size Re-estimation Designs In Confirmatory Clinical Trials—Current State, Statistical Considerations, and Practical Guidance






















No Comments Yet
Let us know what you think