How to calculate blinded sample size re-estimation in clinical trials? Watch our worked example to learn more about this topic and see how blinded SSR may benefit your study.
Adaptive trials enable continual modification to the trial design based on interim data. This means that with adaptive trials, you have the opportunity to make changes to your trial, while it is still ongoing. Which in turn can allow you to explore options and treatments that you would otherwise be unable to which can lead to improvements to your trial, based on data as it becomes available.
How to calculate blinded sample size re-estimation
Below is an example of a blinded sample size re-estimation being carried out in nQuery Clinical Trial Design Platform. It will be an overview, showing you where the advantages and disadvantages of an unblinded sample size re-estimation come into play, in an adaptive clinical trial.
Sample size determination always requires a level of uncertainty over the assumptions made to find the appropriate sample size. Many of these assumed values are for nuisance parameters that are not directly related to the effect size. As such it would be useful to have a better estimate for these values than relying on external sources or the cost of a separate pilot study but without the additional regulatory and logistical costs of using unblinded interim data.
Blinded sample size re-estimation allows the estimation of improved estimates for these nuisance parameters without unblinding the study. The next video will show you an example of a Blinded sample size re-estimation being carried out on nQuery Software using a simple T-Test example. In nQuery you will do a classic sample size determination for a T-test, then apply this to a blinded SSR scenario.
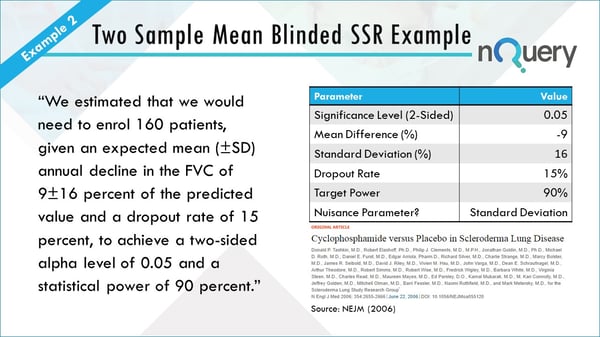
VIDEO: How to calculate blinded sample size re-estimation
This video is an excerpt from our webinar The Advantages & Disadvantages of Adaptive Sample Size Re-Estimation.
What are the advantages of blinded sample size re-estimation?

Earlier Decisions
You have the ability to adjust the study based on an empirical estimate from real data and the real subjects rather than pre-specified estimate for a nuisance parameter.
Reduced Potential Cost
It is cheaper than an external pilot, you save cost and work associated with setting up a completely separate trial before you do your actual trial. There is also the potential to decrease the sample size if your initial nuisance parameter estimate was too high. There is also more chance that your trial will succeed.
Higher Potential Success
Higher success rate means reduced potential cost. The more trials that succeed, the less likely that you’ll have to do a future one. There will be less reliances on conservative guesses which is a major issue for both potential success and cost.
Greater Generalizability
You will have greater powered estimates versus the fixed term but also greater confidence because you’ve dealt with a very obvious objection people might have and the ability to convert to sample size.
Stakeholder Buy-in
It’s a very simple decision which doesn’t have much additional complexity beyond the blinding bias issue. There is no need for a pilot study, so you don’t have to go through the process of getting people to come into your study twice.

This was a relatively simple example of an blinded sample size re-estimation calculation. It highlights the statistical considerations of unblinded SSR and how these could benefit your sample size to ensure your study remains statistically significant.
Like any trial design, careful planning can mitigate or eliminate certain risks.
If you are exploring adaptive designs, one important factor is to select validated and trusted software that is designed for your adaptive trials. nQuery has dedicated adaptive trial design functionality that contains a selection of sample size tables designed specifically for areas of adaptive design.
Learn More About Adaptive Clinical Trials
We recently hosted a webinar examining Advantages & Disadvantages of Adaptive Sample Size Re-Estimation. You can watch this webinar on demand by clicking the image below.

In this webinar you’ll learn about:
- Advantages & Disadvantages of Adaptive Design
- Evaluating Unblinded SSR for your trials
- Evaluating Blinded SSR for your trials
Learn more about Sample Size here























Comments (2)