What are the advantages & disadvantages of blinded sample size re-estimation in clinical trials? Let us quickly examine them and what they mean for you.
The Advantages & Disadvantages of
Blinded Sample Size Re-Estimation
Adaptive trials are any trial where a change or decision is made to a trial while still on-going. It is said that trials with an adaptive design are often more efficient, informative and ethical than trials with a traditional fixed design since they often make better use of resources such as time and money and may require fewer participants.
There are many types of adaptive trials designs. One popular type is that of sample size re-estimation (SSR). A sample size re-estimation (SSR) design is a flexible, adaptive design with the primary purpose of allowing sample size of a study to be reassessed in the mid-course of the study to ensure adequate power.
In the real world, many presumptions at the planning phase are made for the sample size calculation, namely the effect size and any additional “nuisance” parameters (e.g., variability associated with the primary outcome; control group event rate; accrual rate). These can have a level of uncertainty. This uncertainty at the design stage for a confirmatory trial can be mitigated by a sample size re-estimation (SSR) design.
What is blinded sample size re-estimation in clinical trials?
Blinded sample size re-estimation uses interim data without unblinding treatment assignment to provide an updated estimate of a nuisance parameter in order to update the sample size for the trial based on the estimate.
The Advantages & Disadvantages of
Blinded Sample Size Re-Estimation in Clinical Trials
The advantages and disadvantages of blinded sample size re-estimation can be categorized in the same way we treat unblinded SSR. However, the reasons behind reaching these are different. Play the video below to find out why.
This video is an excerpt from our webinar The Advantages & Disadvantages of Adaptive Sample Size Re-Estimation.
The advantages of blinded
sample size re-estimation in clinical trials

Earlier Decisions
You have the ability to adjust the study based on an empirical estimate from real data and the real subjects rather than pre-specified estimate for a nuisance parameter.
Reduced Potential Cost
It is cheaper than an external pilot, you save cost and work associated with setting up a completely separate trial before you do your actual trial. There is also the potential to decrease the sample size if your initial nuisance parameter estimate was too high. There is also more chance that your trial will succeed.
Higher Potential Success
Higher success rate means reduced potential cost. The more trials that succeed, the less likely that you’ll have to do a future one. There will be less reliances on conservative guesses which is a major issue for both potential success and cost.
Greater Generalizability
You will have greater powered estimates versus the fixed term but also greater confidence because you’ve dealt with a very obvious objection people might have and the ability to convert to sample size.
Stakeholder Buy-in
It’s a very simple decision which doesn’t have much additional complexity beyond the blinding bias issue. There is no need for a pilot study, so you don’t have to go through the process of getting people to come into your study twice.
The disadvantages of blinded
sample size re-estimation in clinical trials
Like any trial design, if not executed properly there can be some disadvantages. Below is a quick summary of the potential disadvantages of unblinded sample size re-estimation (SSR). However, there are ways to overcome these with careful planning.
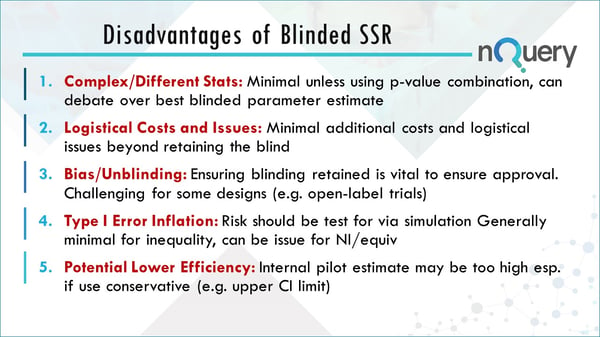
Complex/ Different Stats
Problems here are minimal unless you’re using P-Value Combination, there can be a debate over the best blinded parameter. This could lead to slightly different results.
Logistical Cost and Issues
There are minimal additional costs and very few logistical issues beyond making sure that the blinding is retained and not uncovered.
Bias/Unblinding
You will need to put something in place to stop this unblinding happening. You will need to have a strong justification to show that this won’t be an issue.
Type I error inflation
This is very minimal based on the simulations and papers that are available. For inequality testing in particular it's basically a non-issue.
Potential Lower Efficiency
The internal pilot estimate could be on average a little bit higher than previously if you go for the upper confidence interval limit for that particular estimate, but on average its still better than what you have from not going from the people in your trial.
If you are exploring adaptive designs, one important factor is to select validated and trusted software that is designed for your adaptive trials. nQuery has dedicated adaptive trial design functionality that contains a selection of sample size tables designed specifically for areas of adaptive design.
Learn More About Adaptive Clinical Trials
We recently hosted a webinar examining Advantages & Disadvantages of Adaptive Sample Size Re-Estimation. You can watch this webinar on demand by clicking the image below.

In this webinar you’ll learn about:
- Advantages & Disadvantages of Adaptive Design
- Evaluating Unblinded SSR for your trials
- Evaluating Blinded SSR for your trials

















