What is MCP-Mod?
MCP-Mod (Multiple Comparisons Procedure - Modelling) is an increasingly popular statistical methodology. It can generate superior statistical evidence from Phase II trials with regards to dose selection. The FDA & EMA both recently approved this as fit-for-purpose (FFP).
Update July 2020:
Since its development at Novartis, MCP-Mod promises to devise proof-of-concept and dose-ranging trials with greater evidence and data that can prove critical data for Phase III clinical trial design.
Below is an extract from our webinar Sample Size for Phase II Clinical Trials.
JUMP TO SECTION
What problem is MCP-Mod solving?
Uncertainty about the optimal dose to maximize efficacy and to minimize safety risks remains a major stumbling block on pipeline productivity. A study that examined examined the reasons for the delay and denial of FDA approval during the years of 2000 to 2012, found that this uncertainty was the most frequent reason for the FDA to reject first-time marketing applications for new molecular entities (NMEs).
Rejection may require additional trials, which means lost time for that crucial period of patent protection. Of the NMEs that were subsequently resubmitted after a rejection for any reason, the median approval delay was 14.5 months, ranging up to 6.5 years.
MCP-Mod | Multiple Comparison Procedures-Modelling
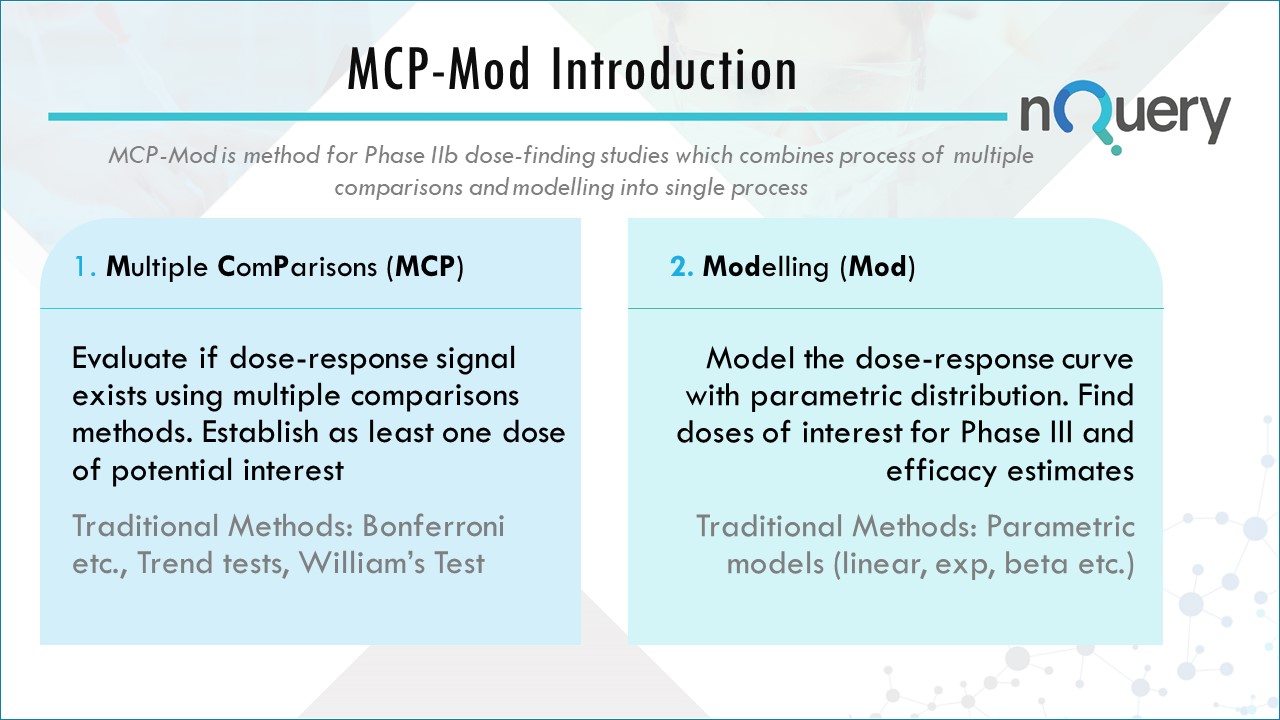
Approved by the FDA & EMA, Multiple Comparison Procedures-Modelling or MCP-Mod is a two-step approach for analyzing Phase II dose-finding data, targeting two of the main Phase II objectives:
1. Establish that the drug works as intended
2. Determine the appropriate doses for Phase III testing
Traditionally, the design and analysis of dose-response studies have been divided into two strategies: one based on multiple comparison procedures (MCP) and another based on modeling. These approaches have their drawbacks however.
Bretz et al. (2005) proposed a methodology for dose-finding studies, called MCP-Mod, combining MCP principles with modeling techniques. Their approach provides the flexibility of modeling for dose estimation, while preserving the robustness to model misspecification associated with MCP.
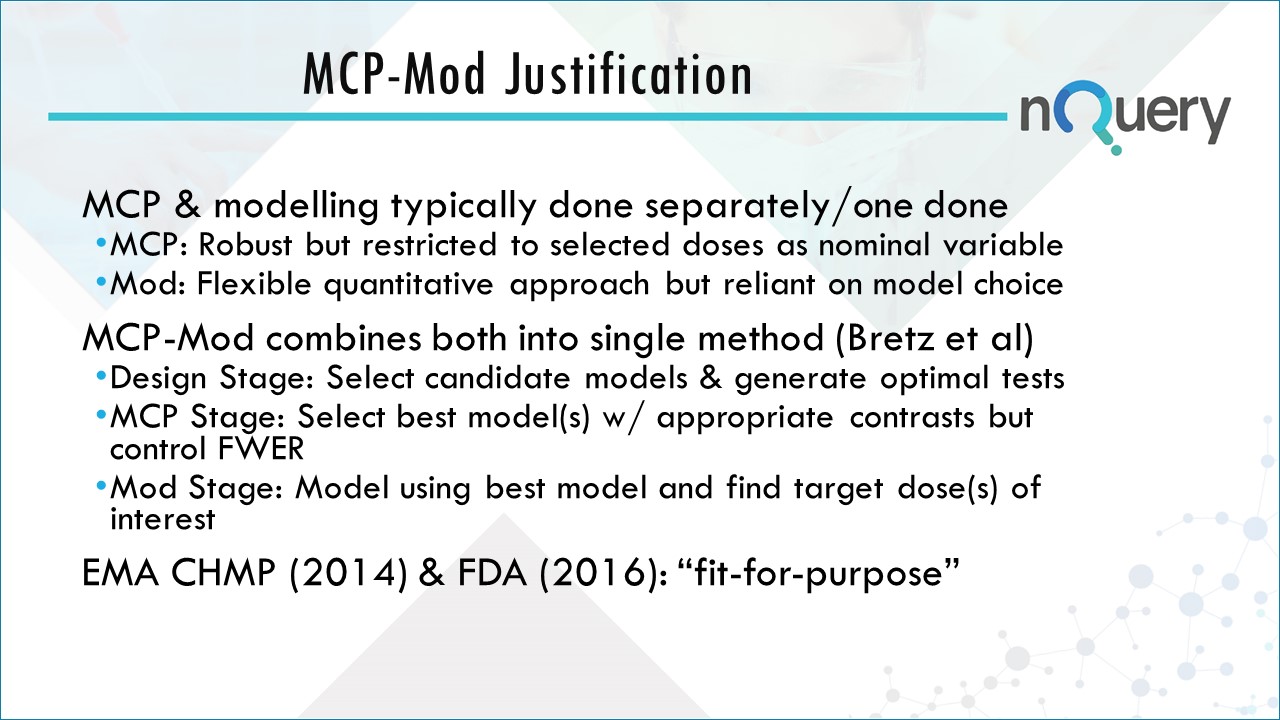
MCP-Mod affects both the design and the analysis of dose finding studies. Traditionally these have been achieved via classical methods. MCP-Mod is technically considered an adaptive analysis tool rather than an adaptive design element.
MCP-Mod provides the added benefit of incorporating the existing uncertainty on the underlying dose-response relation into the study design and analysis method. This results in higher efficiency and greater robustness of the analysis.
The strength of MCP-Mod lies in providing flexibility in characterizing the expected dose-response curve by allowing multiple models to be evaluated at the same time while still giving results which are efficient and control the error rates.
Why use MCP-Mod?
The MCP-Mod approach is efficient in the sense that it uses the available data better than the commonly applied pairwise comparisons.... MCP-Mod represents one tool in the toolbox of the well-informed drug developer.
The European Medicines Agency
FDA Qualification of MCP-Mod
In 2016 the FDA determined that the Multiple Comparison Procedure – Modeling (MCP-Mod) statistical approach is fit-for-purpose (FFP). They have stated that ''the methodology is scientifically sound” and “advantageous in that it considers model uncertainty and is efficient in the use of the available data compared to traditional pairwise comparisons.” You can find more information regarding MCP-Mod from the FDA here.
European Medicines Agency (EMA) &
Approval of MCP-Mod
In 2014, the European Medicines Agency (EMA) qualified MCP-Mod as an efficient statistical methodology for design and analysis of phase 2 dose-finding studies under model uncertainty. You can read the EMA committee report here and their MCP-MOD workshop resources here.
How to use MCP-Mod in clinical trials
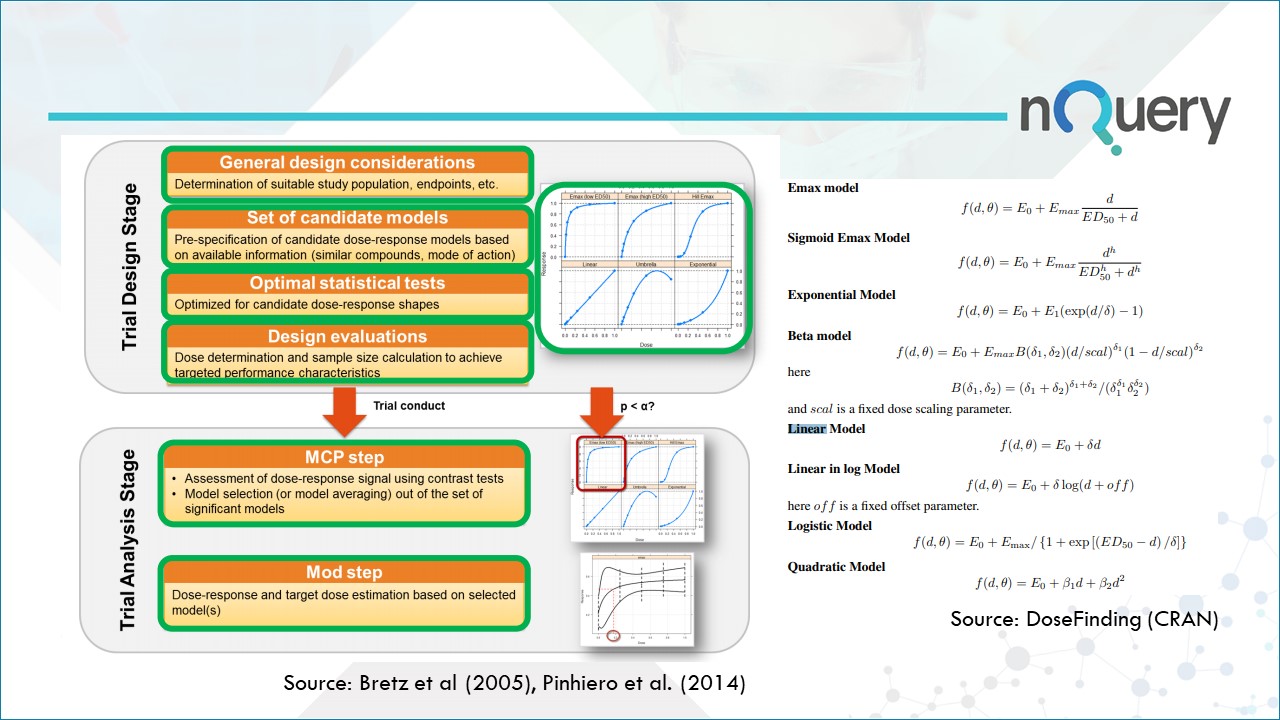
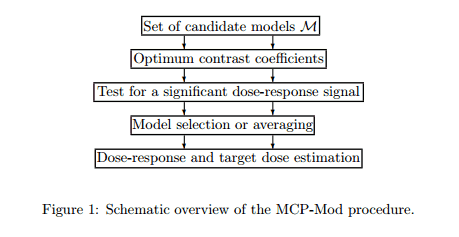
The MCP-Mod procedure consists of 5 steps, but divided across two distinct stages. The contract research organization Cros NT has an interesting blog that lists the steps they take when applying MCP-mod to a trial.
- The first step (MCP-step) is used to assess the presence of a dose response signal using a trend test deducted from a set of pre-specified candidate models.
- The second step (Mod-step) relies on parametric modeling or model averaging to find the “optimal’ dose for confirmatory trials.
Stage 1 - The Trial Design Stage
- Define a suitable study population to represent the underlying true dose-response shape.
- Pre-Specify the candidate dose response models based on available information. These are based on assessment of relevant metrics such as type I error rate, power to detect a significant dose-response shape, and the power to find the minimal effective dose.
- Dose determination and sample size calculation to achieve targeted performance characteristics.
Stage 2 - The Trial Analysis Stage
- Later, a candidate model is selected using model selection criteria like Akaike Information Criterion (AIC) or Bayesian Information Criterion (BIC) or averaging.
- Dose response and target dose estimation based on selected models. (Mod part of MCP-Mod).
Why MCP-Mod is becoming more widely used (Summary)
- According to the European Medicines Agency Qualification Opinion, "The MCP-Mod approach is efficient in the sense that it uses the available data better than the commonly applied pairwise comparisons"
- In 2016, FDA determined that the Multiple Comparison Procedure – Modeling (MCP-Mod) statistical approach is fit-for-purpose (FFP)
- It could reduce costly Phase III failures and post-approval dose adjustments
- It has already been widely implemented and was the primary analysis in 15+ completed studies
MCP-Mod - Case Study
Watch our recent webinar for practical examples of MCP-Mod. This webinar explains the theory behind MCP-Mod and how to plan a study using this method using a practical example. To watch a recording of this webinar and download the slides just click the image below.


















