Statisticians are familiar with the long standing debate regarding Frequentists Vs Bayesian methodologies and the benefits (or negatives) that each present to a particular situation. However, we are now experiencing a rise in traditional frequentists using Bayesian statistics. What are the reasons behind this? While complex, we can identify two high level points.
1) Bayesian Methods Are Becoming More
Common Within Early Stage Clinical Trials
Bayesian statistical methods have become increasingly popular in statistical practice both inside and outside regulated environments. Their growth in popularity can be broadly be attributed to their ability to integrate the following together:
- Prior Information
- Real World Data
- Expert Opinions
The result of this is that it helps create better and more efficient estimates. This has been particularly true in areas such as early stage clinical trials where subjects are often at a premium. While frequentist methods will continue to have a large role in statistical practice, Bayesian methods should now be considered a core part of the working statistician’s toolbox.

2) Bayesian Methods Complement Current
Sample Size Methods
Many Bayes methods in sample size and study planning are being used not to replace current sample size methods but to enhance, complement and more fully explore the results from these methods.
In study planning and sample size estimation, uncertainty over the assumptions required for a sample size determination is a well-known and much discussed issue. However, there are few formal solutions for this problem with an over-reliance on ad-hoc rules for methods such as a sensitivity analysis.
Bayesian methods have been proposed as a method to integrate and explore the effect of this uncertainty when planning a study and finding the appropriate sample size. For example, methods such as Assurance (O’Hagan 2005) provides a method to use expert opinion or previous data to give a more comprehensive idea of the true probability of success for a clinical trial and provide a comprehensive complement to sensitivity analysis.
Other methods such as Mixed Bayesian Likelihood allows researchers to explore the cost of trying to have Bayesian characteristics for the posterior probability while still using a frequentist method for estimation or testing.
All this indicates that Bayesian thinking can be a useful way to formalize and create greater acknowledgment and consideration of the intrinsic uncertainty that comes with trying to find the appropriate sample size for a proposed study.
How Can I Start Integrating Bayesian Methods
Into My Frequentist Work?
To help integrate Bayesian methodology into your clinical trial framework, we have been working closely with our long term big pharma clients, to develop nQuery Bayes - the new nQuery add-on module.
nQuery Bayes provides easy to use sample size tools to allow researchers find an appropriate sample size through formalizing the use of many Bayesian statistical methods such as:
- Bayesian Assurance
- Bayesian Posterior Credibility Intervals
- Mixed Bayesian Likelihood
- Plus other popular Bayesian statistical methods
Quick Example - Bayesian Assurance
Simplified, Bayesian Assurance allows you to get an informative answer on how likely it is to see a “positive” outcome from a trial and then make better decisions on what trials to back. It is complementary to traditional sample size calculations - in that its formalizes sensitivity analysis. Which is pt 5/5 of our "Essential Steps for Sample Size". See below for an example of an assurance calculation in nQuery Bayes.
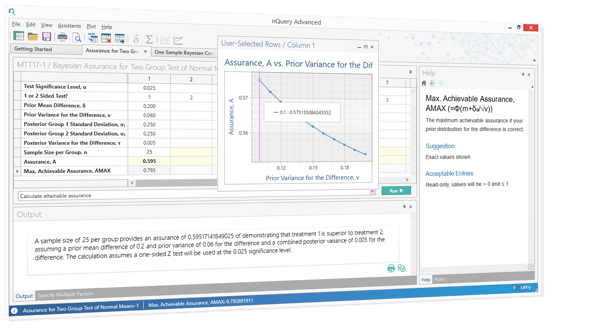
Interested in learning more about Bayesian Assurance?
We recently hosted a webinar Bayesian Assurance: Formalizing Sensitivity Analysis For Sample Size.
You can watch this webinar on demand by clicking the image below.

In this webinar you’ll learn about:
- Benefits of Sensitivity Analysis: What does the researcher gain by conducting a sensitivity analysis?
- Why isn't Sensitivity Analysis formalized: Why does sensitivity analysis still lack the type of formalized rules and grounding to make it a routine part of sample size determination in every field?
- How Bayesian Assurance works: Using Bayesian Assurance provides key contextual information on what is likely to happen over the total range possible values rather than the small number of fixed points used in a sensitivity analysis
- Elicitation & SHELF: How expert opinion is elicited and then how to integrate these opinions with each other plus prior data using the Sheffield Elicitation Framework (SHELF)
- Why use in both Frequentist or Bayesian analysis: How and why these methods can be used for studies which will use Frequentist or Bayesian methods in their final analysis

















