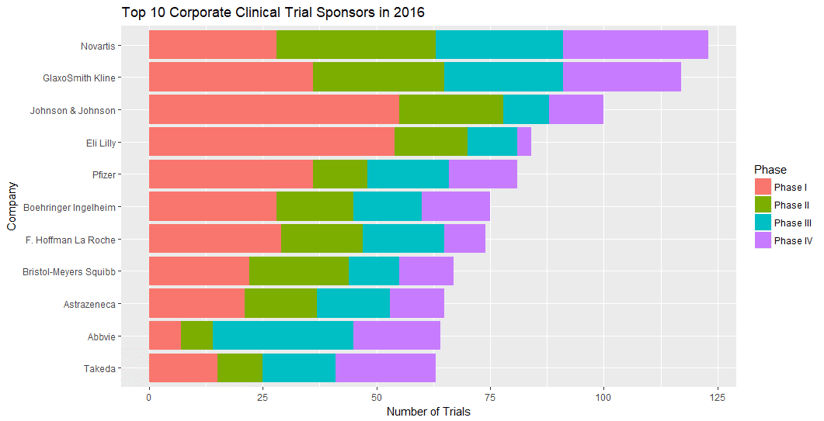
Following on from our previous blog post, The Most Popular Clinical Trial Drugs of 2016, we thought it would be a good idea to take a closer look at the Top 10 Corporate Clinical Trial Sponsors of 2016.
In this post, we explore these Top 10 companies, looking at their developmental histories as well as the drugs they have developed and the number of subjects required for their 2016 registered trials.
Top 10
Corporate Clinical Trial Sponsors of 2016

Novartis is a Swiss multi-national pharma company with headquarters in Basel, Switzerland. It was created in 1996 following the merger of Ciba-Geigy and Sandoz. The company employs over 118,000 people globally and has revenues of almost US$50 Billion. It develops and supplies drugs for the treatment of cancer, cardiovascular diseases, dermatological conditions, neurological disorders, ophthalmic, respiratory & immune diseases, infections and many more. Some of the most popular drugs produced by Novartis include Gleevec (cancer), Diovan (hypertension), Volterol (musculoskeletal) and Cosentyx (Plaque Psoriasis).
Novartis currently sponsors over 3,300 trials across many therapeutic areas and in 2016 alone, Novartis began 123 new trials. It’s plaque psoriasis drug, secukinumab (Cosentyx) had over 10 trials initiated in 2016. Other drugs for which Novartis began multiple clinical trials in 2016 include: Sacubitril & Valsartan (Entresto), Canakinumab (Ilaris), Deferasirox (Jadenu) and Everolimus (Afinitor).
The largest Novartis trial initiated in 2016 has a planned sample size of 14,000, while the smallest trial plans for just 4 subjects. Aggregating planned number of subjects across all clinical in 2016, Novartis estimates that it will require over 52,000 patients in total.

GlaxoSmithKline Plc (GSK) is a British pharmaceutical company, headquartered in Brentford, London. Established in 2000 as a result of the merger of Glaxo Wellcome and SmithKline Beecham, GSK is the world’s 6th largest pharma company (as of 2015). The company manufactures products for major disease areas such as asthma, cancer, infections, diabetes and mental health. Some of GSK’s best known medicinal brands include: Advair, Avodart, Flovent, Augmentin, Lovaza, Lamictal and Paxil/Seroxat.
In 2014, GSK applied for regulatory approval for the first malaria vaccine. Known as RTS,S, the vaccine uses GSK’s proprietary AS01 adjuvant and was developed as a joint project with the PATH Vaccines Initiative and the Bill and Melinda Gates Foundation. GSK has committed to making the vaccine available in developing countries for 5% above production costs.
In 2016, GSK began 117 separate clinical trials, including a large phase 3 study of over 14,500 subjects of its Herpes Zoster Subunit vaccine. Other studies include multiple trials for combined respiratory treatments; Fluticasone Furoate + Umeclidinium Bromide + Vilanterol Trifenatate. In total, over 61,500 subjects will be required by GSK for its trials initiated in 2016, the largest sample size requirement of all the companies in this Top 10 Listing.

Johnson & Johnson (J&J) is an American multinational medical device, pharmaceutical and consumer packaged goods manufacturing company, founded in 1886. Headquartered in New Brunswick NJ, J&J includes some 250 subsidiary companies with worldwide group sales of over $70 Billion in 2015.
J&J’s pharmaceutical business is mainly organized under the Janssen umbrella of companies. J&J’s pharmaceutical products focus mainly on the areas of immunology, neuroscience, infectious diseases and oncology. Immunology products include the anti-tumor necrosis factor antibodies Remicade (infliximab) and Simponi (golimumab) used for the treatment of autoimmune diseases such as rheumatoid arthritis, Crohn’s Disease, ulcerative colitis, etc. In 2013 these 2 products accounted for 29% of J&J’s pharmaceutical revenues and 11.3% of total J&J revenue.
In 2016, J&J began a total of 100 trials that will include almost 16,000 subjects. With a sample size of 5,500, it’s largest trial is a Phase III study for an Ebola virus vaccine. The smallest J&J trial is one of four Phase I trials for Daratumumab, focused on multiple myeloma (Kahler Disease) and features just 6 subjects in total.

An American global pharmaceutical company, Eli Lilly is headquartered in Indianapolis, Indiana. Founded in 1876 by Col. Eli Lilly, the company was the first to mass produce penicillin, the SALK polio vaccine and insulin.
With sales of over US$20 Billion in 2016, Lilly is the 10th largest corporation as measured by global pharmaceutical sales. At the turn of the 20th century, one of Lilly’s great innovations was the use of fruit flavorings for medicines and sugar-coated pills to make their medicines easier to swallow. More recent pharmaceutical developments include; Prozac (fluoxetine) an SSRI for the treatment of clinical depression; Cialis (tadalafil) a competitor to Pfizer’s blockbuster Viagra for erectile dysfunction; Cymbalta (duloxetine) predominantly used to treat major depressive disorders; Gemzar (gemcitabine) for the treatment of pancreatic cancer; and Zyprexa (olanzapine) approved to treat schizophrenia and bi-polar disorder, it is Lilly’s best selling drug of all time.
In 2016, Lilly initiated 84 new clinical trials, the largest being a Phase III study of solanezumab, for the treatment of Alzheimer’s Disease with 2,450 subjects planned. This trial was subsequently terminated due to insufficient scientific evidence that solanezumab would likely demonstrate a meaningful benefit to patients with Alzheimer's Disease.

Pfizer is headquartered in New York City, with its research center in Groton, Connecticut. The company was founded in 1849 by cousins Charles Pfizer and Charles Erhart. In recent times, Pfizer has grown through a number of high-profile corporate acquisitions including: Warner-Lambert (2000), Pharmacia (2003) and Wyeth (2009), the latter being the largest of the three at a cost of $68Billion.
Pfizer has been responsible for introducing many world-class medications such as: Lipitor (atorvastatin) which became the world’s best-selling drug between 1996 – 2012, with more than $125 Billion in total sales; Prevnar (13-valent conjugate Pneumococcal vaccine) that has resulted in an 85% reduction in Pneumococcal infections in children under 5 in the USA; Flagyl (metronidazole) is listed in the WHO List of Essential Medicines and is the drug of choice to treat Clostridium difficle infections; Zoloft (setratine) an SSRI antidepressant used to treat OCD, panic and social anxiety disorders in both adults and children.
In 2016 Pfizer began a total of 81 trials, requiring over 18,000 subjects. 7 of these trials were Phase III studies for Avelumab, the PD-L1 inhibitor developed in conjunction with Merck KGaA. It’s largest trial of 3,000 subjects is a Phase IV study for Tofacitinib Citrate (Xeljanz/Jakvinus) for the treatment of rheumatoid arthritis.

Boehringer Ingelheim (BI) was founded in 1885 by Alfred Boehringer in Ingelheim am Rhein, Germany. Still headquartered in Ingelheim, BI operates a global network of over 146 affiliates with 47,700 employees.
The company’s key drug assets are focused on areas such as respiratory diseases, metabolism, immunology, oncology and diseases of the central nervous system. Brands such as Spiriva Handihaler (tiotrpium, bromide), Striverdi Respimat (olodaterol) both for COPD and Jarsiance (empagliflozin) for diabetes mellitus type II are all from the BI stable of products.
In 2016, BI embarked on 75 new drug trials requiring a total of almost 29,500 subjects. 7 of these trials are focused on nintedanib with trials for idiopathic pulmonary fibrosis as well as a number of oncology-oriented trials. BI’s largest trial features over 7,000 subjects in a Phase IV trial for olodaterol hydrochloride + tiotropium bromide, while the smallest trial of idarucizumab has just 2 subjects.

F. Hoffmann-La Roche (Roche) was founded in 1896 by Fritz Hoffmann-La Roche. The company is headquartered in Basel Switzerland. Descendants of the founder’s families still own approximately 50% of the company, while another third of the shares are owned by fellow Swiss pharma giant Novartis.
Roche was the first company to mass produce vitamin C under the brand name Redoxon and in 1957, it introduced the first generation of Benzodiazepines. Roche’s products span a very wide range of therapeutic areas such as oncology, mental health & sleep disorders, inflammatory diseases, musculoskeletal disorders, cardiovascular illnesses, pain management, HIV/AIDS and many more. Some of Roche’s major branded drugs include Valium, Tamiflu, Naprosyn, Larium, Herceptin, Avastin and numerous others.
In 2016, Roche initiated 75 clinical trials in total, with intentions to recruit just less than 16,000 subjects in total. 13 of these trials were for it’s immunotherapy drug Atexolizumab (Tecentriq).
In 1989, 2 prominent US-based pharma companies, Bristol-Meyers Corp and Squibb Corporation merged to form Bristol-Meyers Squibb (BMS). Both companies had a long history in innovative pharmaceutical development stretching back to the 1850’s. In 1999, US President Bill Clinton awarded BMS the National Medal of Technology, the nation’s highest recognition for technological achievement “for extending and enhancing human life through innovative pharmaceutical research and development and for redefining the science of clinical study through groundbreaking and hugely complex clinical trials that are recognized models in the industry".
Today BMS manufactures prescription pharmaceuticals in several therapeutic areas including cancer, HIV/Aids, cardiovascular diseases, diabetes, hepatitis, rheumatoid arthritis and psychiatric disorders. Its mission is “to develop and define innovative medicines that help patients prevail over serious disease”. Some of BMS brands include Eliquis (apixaban), Plavix (Clopidogrel), Opdivo (nivolumab), Taxol (paclitaxel), Yervoy (ipilimumab), Orencia (abatacept).
In 2016, BMS initiated 67 new clinical trials that required a total sample size of 19,000 subjects. The groundbreaking immunotherapy oncology drug Opdivo (nivolumab) was the target drug in 26 of these trials, either as monotherapy or in combination with other medications.

Astrazeneca is an Anglo-Swedish multinational pharmaceutical company with headquarters in Cambridge, England. The company was formed in 1999 with the merger of Swedish pharma company Astra AB with the UK-based Zeneca Group, which itself was de-merged from ICI in 1993.
Since it’s foundation, Astrazeneca has made several corporate acquisitions such as Cambridge Antibody Technology (2006), MedImmune (2007), and Definiens (2014). Astrazeneca has a portfolio of products for major disease groups including, cancer, cardiovascular, gastrointestinal infection, neuroscience, respiratory, and inflammation. It’s best selling brands include Crestor (rosuvastatin), Symbicort (Budesonide/formoterol), Nexium (esomeprazole), Vimovo (Naproxen/esomeprazole).
In 2016, Astrazeneca undertook 65 new clinical trials, the largest of which is a Phase IV oncology study of osimertinib mysylate (Tagrisso). In total, Astrazeneca expects to recruit 17,708 subjects to satisfy the sample size requirements of all trials in 2016.

Abbvie is a pharmaceutical company that discovers, develops and markets both biopharmaceutical and small molecule drugs. The company originated in 2013 as a spin-off from Abbott Laboratories. As of December 2015, Abbvie employs 28,00 people worldwide.
In 2015, Abbvie’s Rheumatoid Arthritis drug Humira (adalimumab) was the world’s best selling drug, with sales of over $15 Billion. Other brands in Abbvie’s product portfolio include AndroGel (testosterone replacement therapy), Creon (pancreatic enzymes) Duolopa & Duopa (parkinson’s disease) and Synthyroid (levothyroxine).
In 2016 Abbvie registered 64 new clinical trials requiring a total of 15,085 subjects. It’s largest trial is a phase III study of the multiple myeloma drug venetoclax (Venclexta).

Takeda is the oldest company in this list, having been first established in Japan in 1781 (eventually incorporated in 1925). It is the biggest pharma company in Japan and Asia. Takeda first entered the US market in 1977 by developing a joint venture with Abbott Laboratories called TAP Pharmaceuticals. Through TAP, Takeda and Abbott launched the blockbuster drugs Lupron (leuprolide), for the treatment of prostate cancer, breast cancer, emdometriosis & uterine fibroids and Prevacid (lansoprazole) for the treatment of acid reflux and heartburn. In 2008 the TAP joint venture was concluded.
In 2008 Takeda acquired Millenium Pharmaceuticals and established it as an independent subsidiary and later in 2011 Takeda made another high-profile acquisition with the purchase of Nycomed. One of Takeda’s break-through drugs is Actos, used in the treatment of type 2 diabetes. Launched in 1995, Actos became the best selling diabetes drug in the world with annual sales in excess of $5 Billion. Other Takeda best sellers include Dexilcent (dexlansoprazole), Velcade (bortezomib) and Uloric (febuxostat).
In 2016, Takeda initiated 63 new clinical trials across a wide range of therapeutic areas. These trials require a combined sample size of over 22,000 subjects.
Thanks for reading our blog. Here is some further content you may be interested in.
Sample Size FAQs
Answers to the most common sample size and power analysis questions